Cavitation
Let's take a look!
What kind of experiment is this?
Experimental procedure and explanation:
- Attach a clear hose to a faucet. Secure the hose with a hose band or clamp.
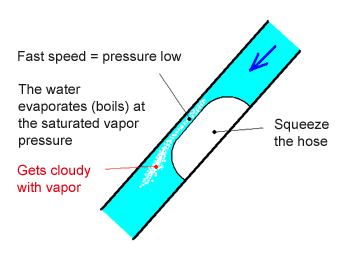
- Squeeze the middle of the hose with pliers. Do not squeeze the hose completely shut, just narrow the flow path. If you are strong enough, you can use your fingers to squeeze.
- Open the faucet and gradually increase the flow. At some point, you will hear a “whoosh” sound and see the water get cloudy. The hose will start to vibrate.
- At this point, part of the water has started to evaporate (boil), creating water vapor.
- Generally with liquids, when the pressure is reduced below the saturated vapor pressure (The pressure at which liquid turns into gas. This depends on the type of material and the temperature.), the liquid starts turning into gas, that is a low-pressure boiling. The phenomenon, which the vapor appears and disappears in short period of time, is called cavitation.
- In this experiment, the flow speed increases where the hose is squeezed, reducing the pressure (the kinetic energy becomes large thereby reducing the pressure), causing cavitation.
| [Keywords] | cavitation, saturated vapor pressure |
| [Reference] | “The Wonders of Flow” Japan Society of Mechanical Engineering, Kodansha Blue Backs pp. 78-83 |
Last Update:9.7.2013

