Newsletter 2023.10 Index
Theme : "AJK FED 2023"
|
Water Condensation in PEMFCs at Nano-scale: Insights through Lattice DFT Simulations
 Clint John Cortes OTIC The University of Tokyo,  Ikuya KINEFUCHI The University of Tokyo, Masazumi ARAO FC-Cubic, Masashi MATSUMOTO FC-Cubic, Hideto IMAI FC-Cubic |
Abstract
In Polymer Electrolyte Membrane Fuel Cells (PEMFCs), Cathode Catalyst Layers (CCLs) play a crucial role in facilitating the oxygen reduction reaction through a network of Platinum (Pt) nanoparticles and ionomer films. Ideally, for optimal catalytic performance, all Pt nanoparticles should be connected to the ionomer network. In recent years, High Surface Carbon (HSC) supports like Ketjenblack have been widely used, where Pt nanoparticles are deposited both on the exterior surface of HSC and within its highly porous interior structure [1]. However, since the ionomer cannot penetrate micro-pores, Pt nanoparticles deposited inside HSCs remain disconnected from the ionomer network. Nevertheless, the use of HSCs has significantly improved PEMFC performance. This seemingly counter-intuitive result can be explained by water condensation. Condensed water acts as a conductive pathway for protons, forming water bridges that link interior Pt nanoparticles to the exterior Pt-ionomer network [2]. Investigating water condensation inside the micro-pores of a CCL particle is challenging due to the system's extremely small scale. Visualizing nano-scale water condensation through experiments is tediously difficult while remaining too large for molecular-based simulations.
In this study, we employed a coarse-grained numerical simulation using reconstructed structural data from an actual CCL particle sourced from a commercial PEMFC, specifically the Toyota MIRAI Gen 2 model. Firstly, the porous structure of the CCL particle is reconstructed from segmented images (Pt nanoparticles, carbon support, and void/pores) taken by Transmission Electron Microscopy (TEM) with a resolution of 0.18nm, as shown in Figure 1 (a) – (b).
The water condensation is reproduced using the lattice Density Functional Theory (DFT) simulation, a computationally efficient approach to study phase changes in complex porous structures [3]. To validate the parameters used in our lattice DFT simulations, we compared the isotherm curve obtained from our simulation results with experimental data. A sample of the resulting water distribution (after parametric analysis) is shown in Figure 1 (c).
The resulting water distribution obtained from the simulation serves as crucial input to investigate Pt nanoparticle activation under varying relative humidity (RH) conditions. Specifically, we imposed criteria for the activation of Pt nanoparticles based on the presence of continuous paths of condensed water (known as 'water bridges') that connect interior Pt nanoparticles to the outer surface of the CCL particle, as shown in Figure 2. In Figure 1 (c), activated Pt nanoparticles are colored red, inactive ones are colored yellow.
We demonstrated in this study that lattice DFT simulation can be a powerful yet less expensive tool to reproduce water condensation in CCL particles of PEMFCs, and at the same time ascertain Pt activation behavior at different RH conditions. Although there is still room for further investigation on this topic, this study can help shed light on optimizing Pt nanoparticle utilization for enhanced fuel cell performance.
Acknowledgment
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) of Japan (Grant number JPNP20003).
Key words
polymer electrolyte membrane fuel cell, cathode catalyst layer, lattice density functional theory, capillary condensation of water, porous media
References
Figures
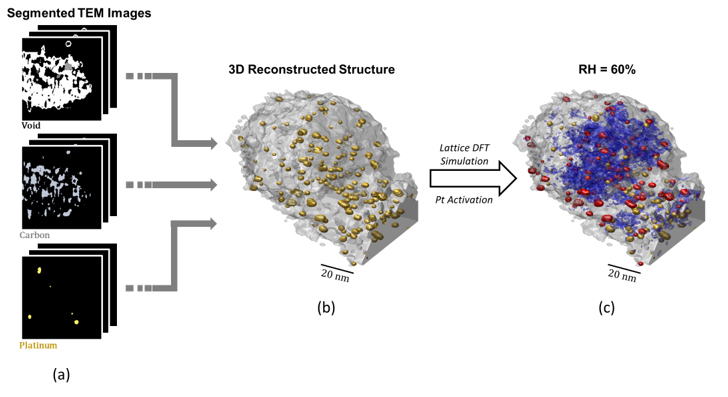
Fig. 1 (a) Segmented TEM images of a PEMFC cathode catalyst layer particle used in Toyota MIRAI Gen 2 model. (b) 3D Reconstructed porous structure from TEM images. (c) Distribution of condensed water (blue) obtained from lattice DFT simulation, with corresponding activated Pt (red) and inactive Pt (yellow).
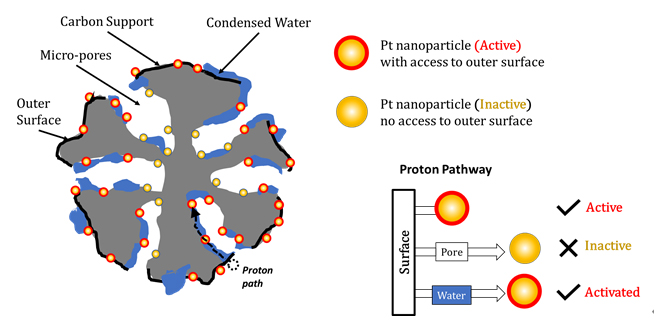
Fig. 2 Schematic of activation of Pt nanoparticles based on possible proton pathway.

