Which is Heavier, Water or Stone? (What is Density?)
Let's take a look!
What type of experiment is this?
Experimental procedure and explanation:
- In this experiment, I will apply the volume measurement method using buoyancy introduced in “Measuring Volume 1.”
- To determine which is heavier—water or stone—I will place a stone in water.
- The stone sinks, suggesting it is heavier than water.
- However, is the stone truly heavier than the water?
The stone weighs 33.7 g, while the water in the container weighs 250 g—meaning the water is heavier. So, what’s going on? - Because the weight of water depends on its amount, comparing the total weights alone cannot determine whether an object will float or sink.
- To determine whether an object will float or sink, you need to compare the weights of objects with the same volume—not their total weights.
- This comparison is done using density, defined as (Density) = (mass) ÷ (volume). (Note: On Earth, mass and weight can be treated as equivalent for practical purposes. For example, an object weighing 100 g has a mass of 100 g.)
- Density allows us to compare substances to predict if an object will float or sink in water.
- Measure the density of the stone you used. Next, measure the density of the stone used in this experiment by determining its volume using the method described in “Measuring Volume 1.”
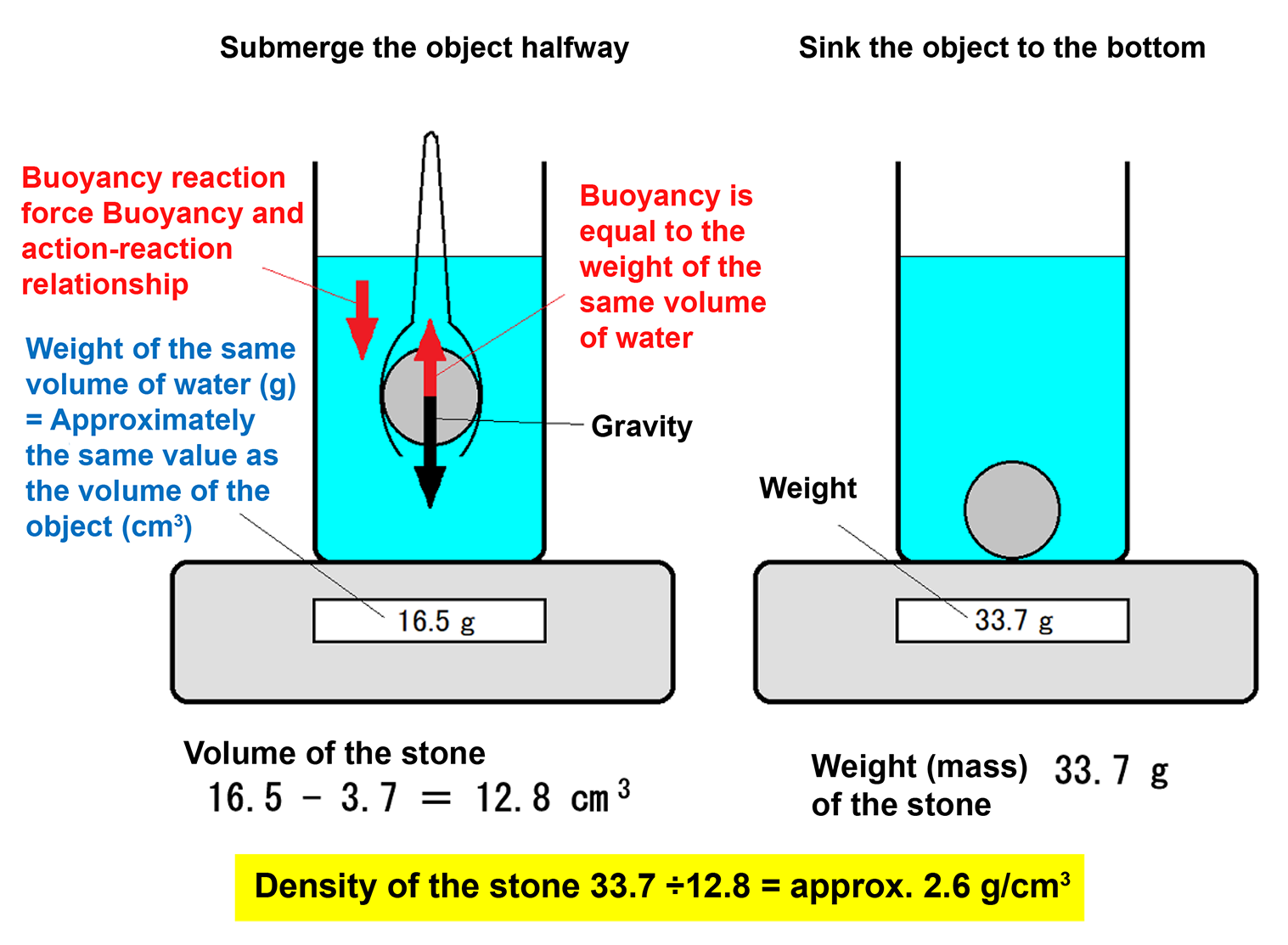
- Place a container filled with water on a scale and reset it to zero (tare), so the display reads 0 g.
- Submerge the stone (or tongs holding it) up to the marked line and measure the increase in weight. For example, if the scale reads 3.7 g, the displaced water volume is approximately 3.7 cm3.
- Next, measure the volume of the stone by partially submerging it in water. For example, if the scale reads 16.5 g when the stone is submerged halfway, subtract the tongs’ volume (3.7 g) to get the stone’s volume: 16.5 − 3.7 = 12.8 cm3.
- Then, fully submerge the stone so it rests at the bottom. The scale reading at this point represents the stone’s weight (mass). For example, if the scale reads 33.7 g, then the stone’s mass is 33.7 g.
- Using these values, the stone’s density can be calculated as follows:
(Density) = (mass) ÷ (volume) = 33.7 (g) ÷ 12.8 (cm3) = approximately 2.6 (g/cm3). - Since the density of water is approximately 1.0 g/cm3, any object with a density greater than this will sink. Therefore, the stone “is heavier than water” means it has a higher density than water.
- In summary, density is the property used to compare the weights (masses) of substances of equal volume.
- This video was produced with the support of the JSPS Grant-in-Aid for Scientific Research (18K03956).
| [Keywords] | Buoyancy |
| [Related items] | Volume Measurement, Measuring Volume 1 |
| [References] | Ryozo Ishiwata and Mitsumasa Nemoto, “The Wonder of Flow,” Kodansha Bluebacks, pp. 48–51. Ryozo Ishiwata, “Illustrated Fluid Dynamics Trivia,” Natsume Publishing, pp. 188–189. |
Last Update: 2021.8.1

