Is the Gold Medal Genuine?
Let's take a look!
What type of experiment is this?
Experimental procedure and explanation:
- Find out if your gold, silver, or bronze medal is genuine.
- You can determine this by checking the density. Let’s measure the density of each medal using the method described in “Which is Heavier, Water or Stone?”
- Place a container filled with water on the scale and reset the display to zero (0 g).
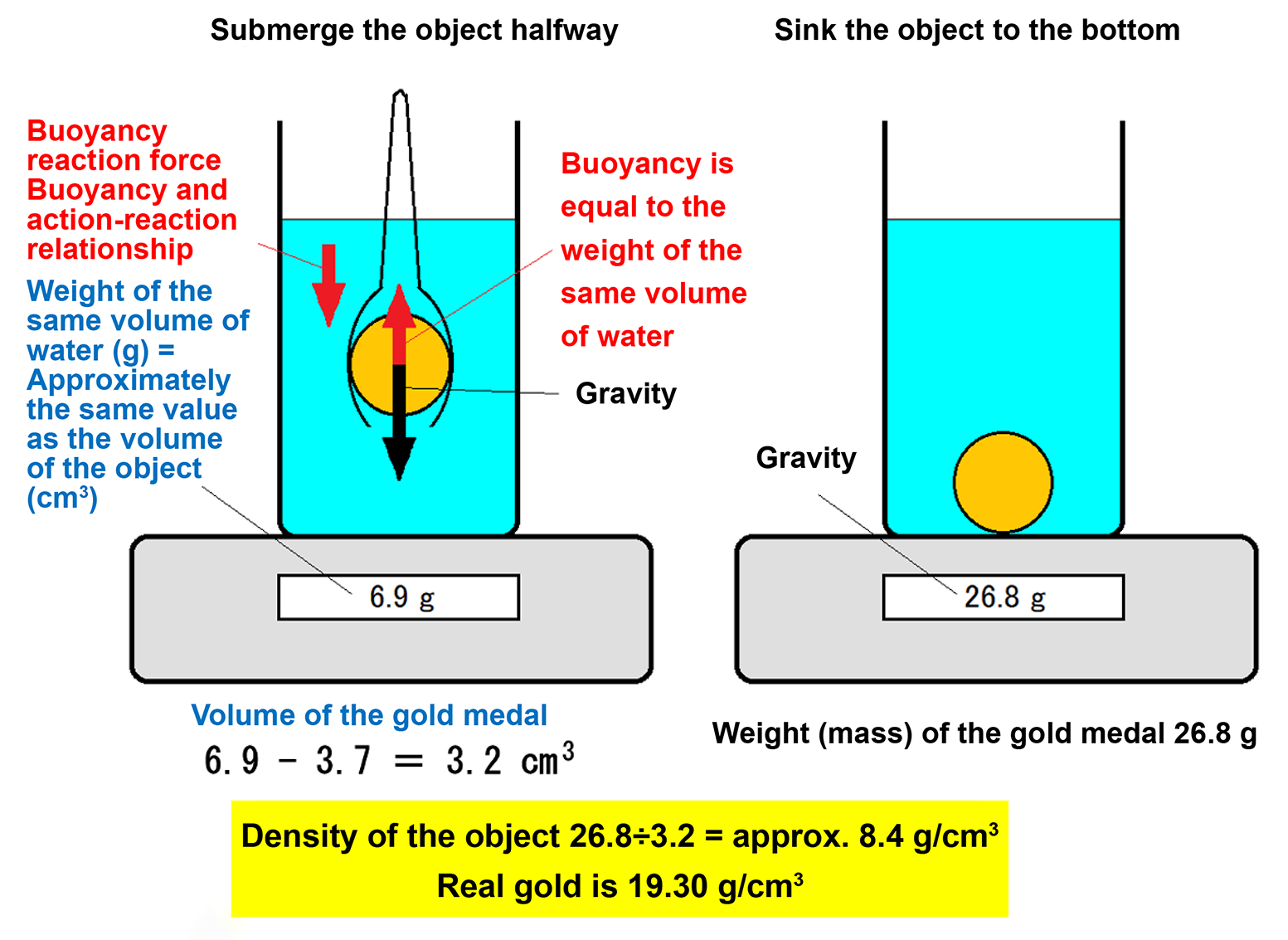
- Submerge the medal halfway to measure its volume, then fully submerge it to the bottom to measure its weight (mass).
- For example, the gold medal’s volume reading is 6.9 cm3 (including 3.7 cm3 from the tongs), and its weight (mass) is 26.8 g. Calculate the medal’s density as (Density) = (mass) ÷ (volume) = 26.8 (g) ÷ (6.9 − 3.7) (cm3) = approximately 8.4 (g/cm3).
- Since the density of pure gold is 19.30 g/cm3, this medal is not genuine gold—it is simply gold-colored.
- The volume of the silver medal is 6.8 cm3 (including 3.7 cm3 from the tongs), and its weight (mass) is 26.6 g. Calculate the density as follows: (Density) = (mass) ÷ (volume) = 26.6 (g) ÷ (6.8 − 3.7) (cm3) = approximately 8.6 (g/cm3).
- Since the density of pure silver is 10.49 g/cm3, this medal is not genuine silver.
- The volume of the bronze medal is also 6.8 cm3 (including 3.7 cm3 from the tongs), and its weight (mass) is 27.9 g. Calculate the density as follows: (Density) = (mass) ÷ (volume) = 27.9 (g) ÷ (6.8 − 3.7) (cm3) = approximately 9.0 (g/cm3).
- Since copper has a density of 8.96 g/cm3, this medal is most likely made of copper or a copper alloy.
- Given that all three medals have densities close in magnitude, it is reasonable to assume that copper is the main component in all of them.
- This video was produced with the support of the JSPS Grant-in-Aid for Scientific Research (18K03956).
| [Keywords] | Buoyancy |
| [Related items] | |
| [References] | Ryozo Ishiwata and Mitsumasa Nemoto, “The Wonder of Flow,” Kodansha Bluebacks, pp. 48–51. |
Last Update: 2021.8.1

