Rock Density
Let's take a look!
What type of experiment is this?
Experimental procedure and explanation:
- Measure the density of various rocks. Measure it in the same way as described in “Which is Heavier, Water or Stone?”
- Place a container filled with water on the scale and reset the display to zero (0 g).
- Submerge the rock halfway to determine its volume, then fully submerge it to measure its weight (mass).
- The volume of the granite, including 3.7 cm3 from the tongs, is 74.7 cm3, and its weight (mass) is 184.4 g.
The density is calculated as
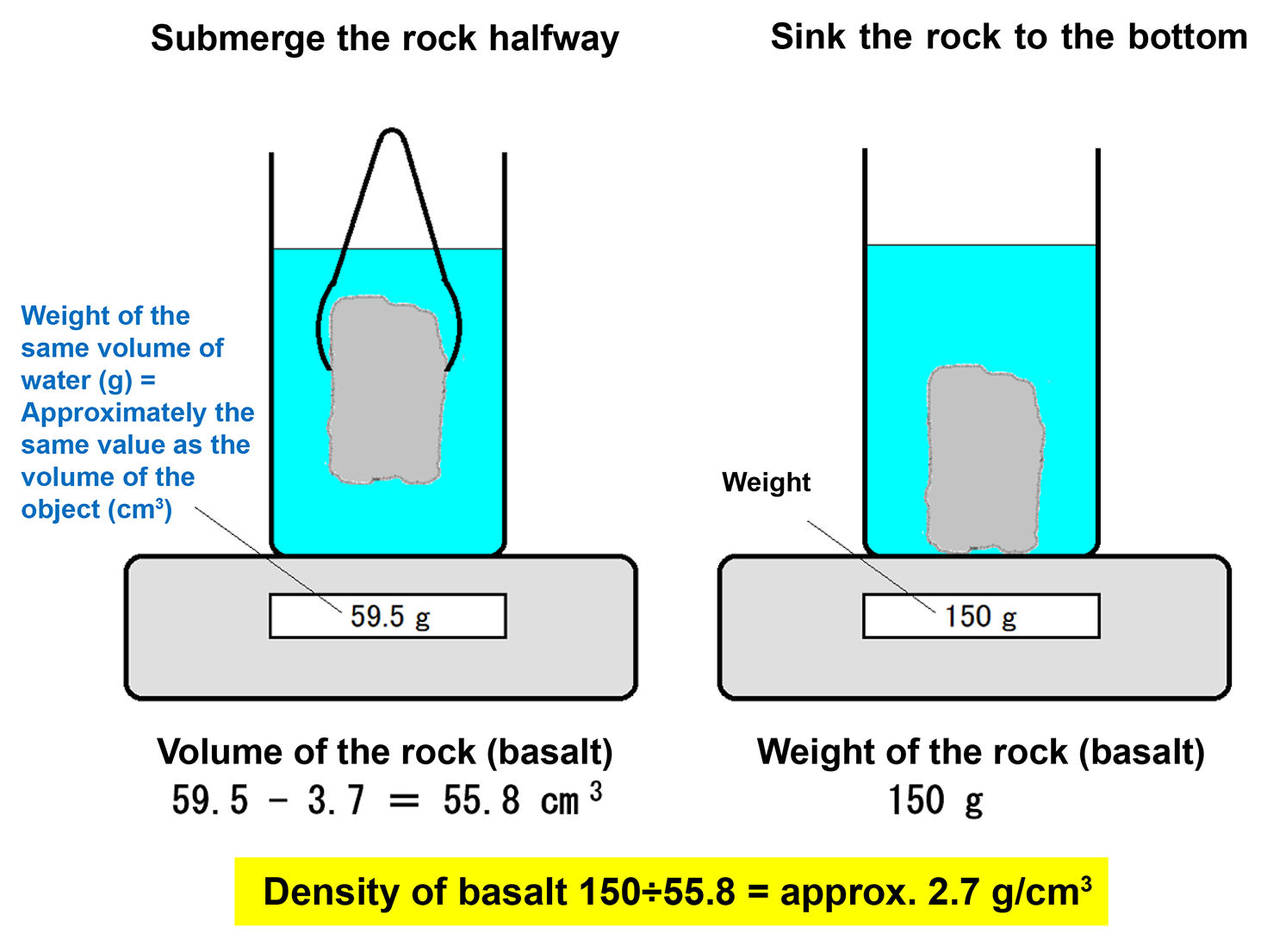
(Density) = (mass) ÷ (volume) = 184.4 (g) ÷ (74.7 − 3.7) (cm3) = approximately 2.6 (g/cm3). - The volume of the basalt is 59.5 cm3 (including 3.7 cm3 from the tongs), and its weight (mass) is 150.0 g.
The density is calculated as
(Density) = (mass) ÷ (volume) = 150.0 (g) ÷ (59.5 − 3.7) (cm3) = approximately 2.7 (g/cm3). - The volume of the crystal is 15.3 cm3 (including 3.7 cm3 from the tongs), and its weight (mass) is 30.8 g.
The density is calculated as
(Density) = (mass) ÷ (volume) = 30.8 (g) ÷ (15.3 − 3.7) (cm3) = approximately 2.7 (g/cm3). - The crystal used here is quartz.
- Try measuring the density of various materials—not just rocks.
- This video was produced with the support of the JSPS Grant-in-Aid for Scientific Research (18K03956).
| [Keywords] | Buoyancy |
| [Related items] | |
| [References] | Ryozo Ishiwata and Mitsumasa Nemoto, “The Wonder of Flow,” Kodansha Bluebacks, pp. 48–51. Ryozo Ishiwata, “Illustrated Fluid Dynamics Trivia,” Natsume Publishing, pp. 188–189. |
Last Update: 2021.8.1

