Newsletter 2023.2 Index
Theme : "The Conference of Fluid Engineering Division (February issue)”
|
Development of Innovative PEDOT Synthesis Using Plasma Enveloped Bubble
|
Abstract
Poly(3,4-ethylenedioxythiophene) (PEDOT) is known as one of the best conducting and already applied to many electronic devices. PEDOT is typically synthesized by chemical oxidation. For enhancement of PEDOT solubility, Poly(styrenesulfonate) (PSS) is required as dopants which suppresses electrical conductivity.
In this study, the innovative plasma polymerization process was developed utilizing plasma enveloped bubble. Plasma polymerization is a promising method for PEDOT synthesis without any dopants or chemical oxidation agents. In the plasma polymerization, the polymerization is initiated by the reactive radicals with high oxidation potential generated by the discharge n bubbles. By applying this plasma enveloped bubble technique to uniformly dispersed EDOT monomer in water (EDOT emulsion), the PEDOT synthesis with high water dispersion can be realized without introducing any chemical dopants.
For clarifying the effectiveness of processing EDOT emulsion with plasma polymerization by discharge inside bubbles, the material characteristics of the synthesized PEDOT from EDOT emulsion and pure EDOT were experimentally elucidated for different oxygen concentration in the plasma forming gas. The experimental setup in this study is shown in Figure 1.
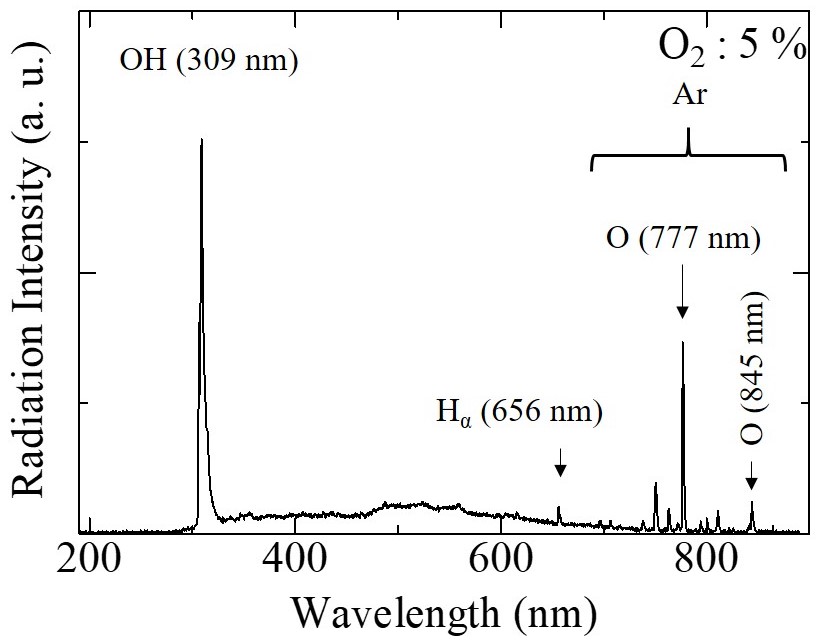
Figure 2 shows the emission spectra from discharge inside bubbles at 5 % and 50 % of oxygen concentrations. The lower oxygen concentration results in the reduction of OH radical with increasing of the O radical composition ratio.
Photographs of the processed samples for each oxygen concentration are shown in Figure 3. Through this method with the plasma enveloped bubble, the processed samples with high transparency and water dispersibility are successfully obtained. Figure 4 shows the conductivities of the elaborated films from the processed liquid samples. The PEDOT film with the highest electrical conductivity was obtained at 5% of oxygen concentration. This is because the enhancement of polymerization and doping occurred due to the increase of composition rate of O radicals with suppressing the degradation of polymer molecular structure.
Key words
Plasma polymerization, PEDOT synthesis, Discharge inside bubbles, Conducting polymer, Radical generation
Figures
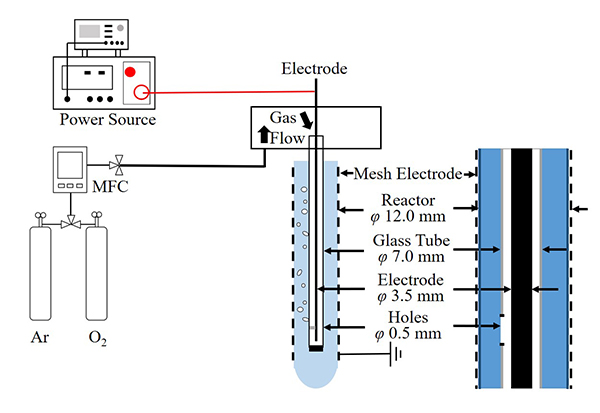
Fig. 1 Schematic illustration of experimental setup for EDOT polymerization with discharge in bubbles.
 |
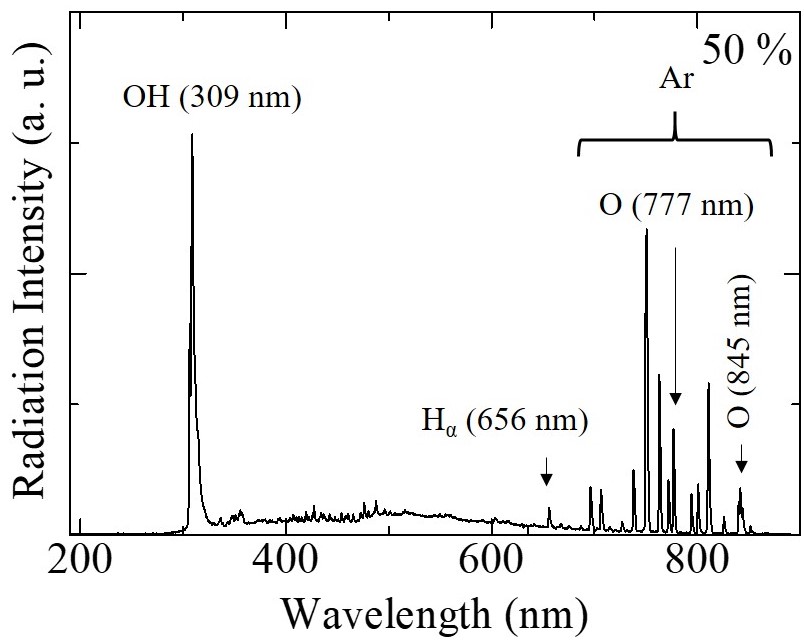 |
| Fig. 2 Emission spectra from discharge for 5 % and 50 % of oxygen concentrations. | |
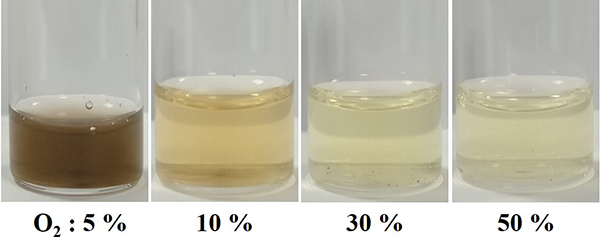
Fig. 3 Pictures of synthesized water dispersing PEDOT for different oxygen concentrations.
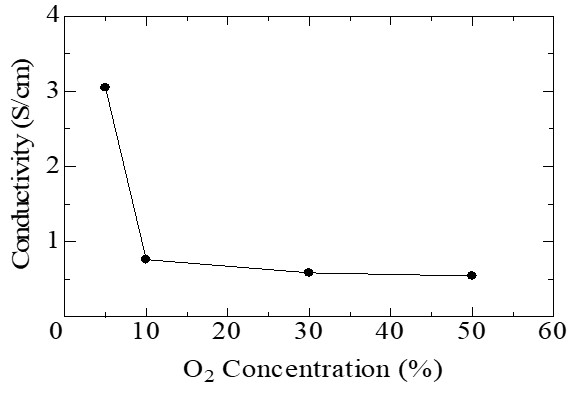
Fig. 4 Electrical conductivities of synthesized PEDOT films for each oxygen concentrations.



